Last updated 6th July 2021.
This article explains the result from the Roche Anti-SARS-CoV-2 (S) test to give Testing For All users a better understanding of how this diagnostic test works. The aim of this product is to help the Testing For All community to understand their immune response, and hence their immunity status to COVID-19. For those that wish to, this test can also be used to monitor your antibody level (or antibody titer as it is more formally known) over time as the test provides a calibrated result in U/mL that will change as your IgG antibody level builds and then wanes over an extended period of time.
The body has a range of ways to fight off infection, but the scientific community has centred the development of vaccines for COVID on triggering the creation of antibodies to the spike protein of coronavirus. These “spikes” are what gives coronaviruses their name, and it is through this spike mechanism that the virus binds to healthy human cells in order to replicate itself. A natural infection creates a wide ranged defence, often involving a T cell response to destroy human cells infected by SARS-SoV-2 to stop virus from replicating, and by the creation of a range of antibodies to different proteins in the virus that attempt to deactivate it before it can bind to host cells. The original COVID-19 Home Total Antibody Test we launched in Sept 2020 detects antibodies to the Nucleocapsid protein that surrounds the RNA of the coronavirus, and is useful for detecting previous exposure, which has provided many people with an understanding of whether past or ongoing symptoms that they experienced were related to a coronavirus infection that occurred before PCR testing was widely available.
The COVID-19 Immunity Test provides an accurate calibrated measurement of the level of antibodies to the spike protein, and is therefore considered to be a good view of your immune response and status. In the testing that we have carried out with our laboratory partner, we have compared the result of the both the Roche N and S test tests on positive blood samples and see very good agreement so if you tested positive on our COVID-19 Home Total Antibody Test then you will very likely test positive on the COVID-19 Immunity Test. The difference between the two tests is that they are looking at different viral protein targets, and that the Immunity Test gives you a calibrated antibody titer that can be compared to other tests and the emerging WHO standard.
What is the range of results?
The Roche Anti-SARS-CoV-2 (S) test will return “< 0.4” (less than o.4) in the case where the response is below the instrument’s limit of detection, meaning that this sensitive instrument could not detect any neutralising antibodies. This is the most common result for negative responses, with 97% of negative tests to date returning this result.
Positive results are greater than 0.8, with the upper bound listed as “> 2,500” or greater than 2,500. Those that receive a result of > 2,500 have a neutralising antibody response of at least 2,500 U/mL indicating a very high antibody level.
In the period 12th Feb to 12th June 2021 Testing For All completed 3,016 quantitative Anti-SARS-CoV-2 (S) tests. The range of results is shown below:
| Result Type | Level (U/mL) | Number of Results |
| Negative – Antibodies not found | < 0.4 (less than 0.4) | 646 |
| Between 0.4 and 0.8 | 21 | |
| Positive – Antibodies found | Between 0.8 and 10 | 232 |
| Between 10 and 100 | 702 | |
| Between 100 and 250 | 325 | |
| Between 250 and 1,000 | 381 | |
| Between 1,000 and 2,500 | 246 | |
| > 2,500 (more than 2,500) | 463 |
The sections below will be updated shortly to include the break down by number of doses, and immune status.
Protection following vaccination
One of the primary use cases for this test is to check that your immune system has responded correctly to the vaccine and produced antibodies in the nominal range. Research indicates that vaccination can fail due to either the way the vaccine was manufactured and handled, or due to the host’s immune response. Testing For All will continue to publish data on the immune response created by the different vaccines available in the UK.
The two main vaccines available in the UK: Pfizer/BioNTech and AstraZeneca, both aim to create IgG antibodies to the spike protein of this coronavirus. The level of antibodies created by your body following the vaccine is measured using this test. The primary way the effectiveness of a vaccine is assessed is through its “efficacy.” This can be explained by imagining that 100 people are ill with COVID. “90% efficacy” means if only they’d had the vaccine, on average only 10 would have got ill. Vaccine efficacy is the relative reduction in the risk: whatever your risk was before, it is reduced by 90% if you get vaccinated. There is a lot of confusion about this number: it does not mean there is a 10% chance of getting Covid-19 if vaccinated – that chance will be massively lower than 10%. Researchers estimate efficacy by comparing numbers of new cases in vaccinated and unvaccinated people through a randomised trial. In addition, public health bodies around the world are monitoring “real-world” efficacy by monitoring those that have been vaccinated.
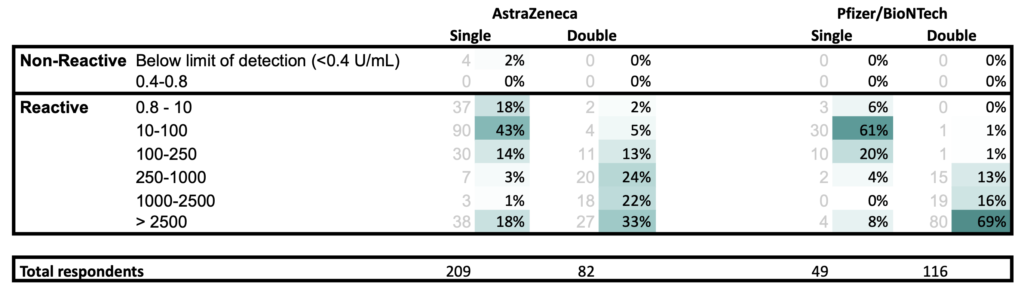
For those who answered “No” to “Are you diagnosed or believed to be immunocompromised?“
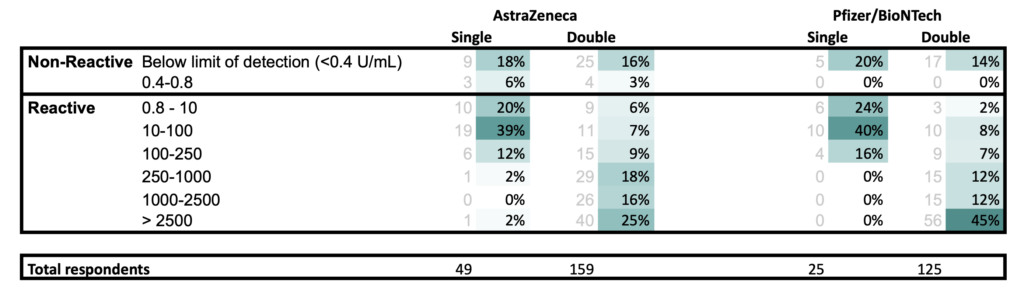
For those who answered “Yes” to “Are you diagnosed or believed to be immunocompromised?“
Notes:
- All results at least 21 days post vaccination
- Respondents for single and double vaccination may or may not be the same person
Johnson & Johnson, Moderna and Novavax
At this time we do not have enough result information to publish data for these vaccines.
Protection following natural infection
From the data that Testing For All users shared through the Antibody Duration and Immunity Survey we can see that the antibody response created by natural infection varies with the symptoms experienced. Those that experienced no symptoms or mild symptoms that do not affect one’s daily life may not produce detectable IgG antibodies, whereas moderate and severe infections typically do. Mild or asymptomatic infections also lead to a slower build up of antibodies, that may only be detectable after 6 or 8 weeks or potentially longer.
There is growing research both from the Antibody Duration and Immunity Survey and wider research that a natural infection does provide a level of protection against reinfection. Like most medical matters this is unfortunately not guaranteed and there will always be edge cases.
Typical result following natural infection ~ 0.8 – 250 U/mL based on analysis of 72 samples.
Your result
If you have taken the test, you can see your interpretation at https://testingforall.app/tests
Frequently Asked Questions
Please revisit this page in the future, as we will continue to add further information about protection, antibody build up and duration and the evolving picture on reinfection and immunity.
I have a negative response following vaccination. What should I do?
If you have waited 21 days following vaccination and tested negative this is a strong indicator that you have not had the intended immune response. This may be due to an issue with the dose that you were provided, or some other factor to do with how the vaccine works. The good news is that a second dose consistently gives a stronger immune response and there are different vaccine types (eg. AstraZeneca as an adenovirus vector, and Pfizer using messenger RNA which are completely different technologies).
The MHRA currently operates a dedicated Yellow Card service for tracking any adverse incidents related to coronavirus treatment, however this service does not allow you to register not having an expected reaction. Testing For All recommends completing a defective product report if you test negative after 21 days post vaccination, and have no mitigating circumstances such as ongoing immunosuppressant therapies such as cancer treatment. A template for the required information is provided here.
We also recommend to speak with your GP to enquire if you are eligible for a second dose if you have only received a single dose to date. If you wish, you can also book a GP appointment with HealthHero via the test result page in testingforall.app however they will not be able to arrange additional vaccination at this time.
My positive interpretation of 0.8-10 U/mL following vaccination feels low. Should I be worried?
The most important thing is that your body is shown to have responded to the vaccine by creating neutralising antibodies. A study and by Public Health Scotland across 1.4m individuals shows that having had a vaccine greatly reduces your chance of becoming seriously ill and being admitted to hospital. At this time it is not clear whether the level of antibodies directly equates to the level of protection. The most important thing is that you have “seroconverted” and created a measurable antibody response.
My negative interpretation of “< 0.40” is the same as my friend’s interpretation. Is this right?
The majority of negative interpretations are “< 0.40” which means that the instrument was not able to detect neutralising antibodies.
Links to Additional Research
This article attempts to explain simply how the sophisticated analytical diagnostic returns its results. For further reading on research related to anti-SARS-CoV-2 antibodies please see the following links:
Study showing COVID-19 antibodies remain for at least 6 months by UK Biobank
Coronavirus antibody tests: what we know so far by The Crick’s George Kassiotis
Immune responses and immunity to SARS-CoV-2 by the European Centre for Disease Prevention and Control
James Monico
Co-Founder, Testing For All
